The systematic construction of specific architectures from functional molecular building blocks has been an area of active research over the past few years. Examples range from coordination polymers, to metal oxide/organic nanocomposites, to larger cluster-based building blocks. Coordination network materials are of interest in applications involving specific guest adsorption, catalysis, separation, ion exchange, and uses which depend on the magnetic and electronic properties of single metal centers. The production of kinetically stable, crystalline networks requires that the bonding interactions be strong enough to connect the units, but also labile enough to permit reorganization into ordered, crystalline structures. The challenge of such "crystal engineering" is trying to predict the outcome of the structure. Small modifications of the synthetic technique, such as a change in the solvent or counterion, can lead to different products. By employing geometrically rigid clusters, with a set number of bonding sites and directional arrangements, as building blocks allows a certain degree of control over the architecture of the crystalline product.
Organically modified polyoxometalate (POM) clusters of the types [Mo12As4O46(R)4]4- or [W6As2O25H(R)2]5- contain multiple organic groups that can be tailored and used as directional linking sites. The [Mo12As4O46(R)4]4- clusters possess a tetrahedral arrangement of organic groups, while the [W6As2O25H(R)2]5- clusters have an approximately linear arrangement of organic groups (see Figure). Clusters of these varieties containing a number of different organic functionalities were used as molecular building blocks for the formation of hybrid POM-based polymeric structures. By controlling the functionality of the organic groups, the choice of solvent, and the counterion, interesting crystalline architectures may be formed (see Figure). Coordination linkages can form between counterions and cluster oxo ligands or organic groups, and hydrogen-bonding linkages can form between organic groups and oxo ligands or organic groups on adjacent POMs. When linking occurs between the organic functional groups on adjacent POM clusters, open framework structures can form.
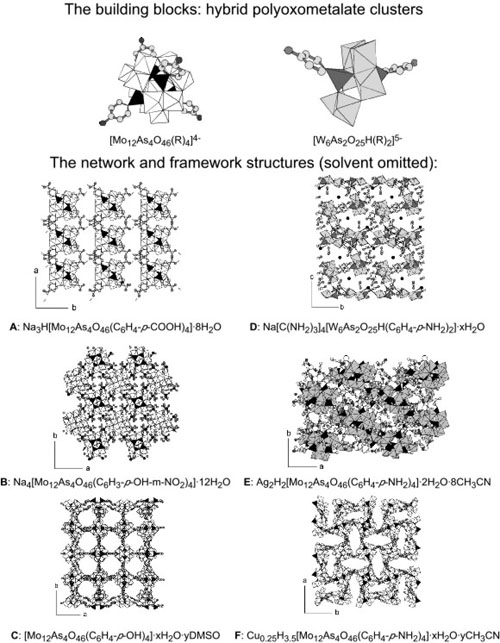
Interactions between the organic groups, the solvent molecules, and neighboring clusters all affect the final solid-state architecture and connectivity. The connectivity and packing of the clusters depended on the hydrogen bonding capability of the organic groups and solvent, and the affinity of the counterions for the POM. In compound (A), intercluster hydrogen bonding between carboxylic acid groups resulted in the formation of stacked 2D nets. In compound (B), intermolecular hydrogen bonds were not observed, and a close-packed structure was formed. In compound (C), hydrogen bonding between phenol groups and oxo ligands combined with the absence of bonds between counterions and organic groups, and a solvent that was not involved in hydrogen bonding, yielded an open framework structure. In compound (D), both Na+ and [C(NH2)3]+ ions formed bonds with the cluster oxo ligands, linking the POM clusters into tetrameric units and 1D chains. In compound (E), interactions between Ag+ and both the oxo ligands and the aniline groups of the POM linked the clusters into 1D chains. In compound (F), hydrogen-bonding interactions between the clusters led to an open structure. While the goal of tailoring the crystal structures of organically modified POM clusters to have predictable geometries has yet to be realized, it is hoped that an understanding of some parameters affecting the crystallization will allow the production of more open-framework or porous hybrid materials.
Open framework structures of this variety may have applications as ion-exchange materials due to the high charges of POMs, although we still need to demonstrate such applications. The crystalline networks based on the above clusters cannot be directly applied as redox catalysts, since this particular type of POM has irreversible redox reactions. However, by applying some of the synthetic principles developed in this research, materials with catalytic activity, host-guest interactions, and other unique reactivity might be created by utilizing redox active, electronic, or magnetic POMs as the molecular building blocks.