Synthesis of Ordered Macroporous Metal Oxides, Metals, and Hybrid Solids
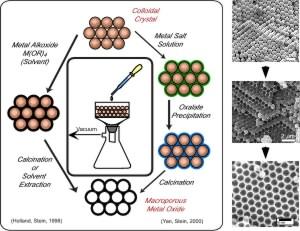
We have developed colloidal crystal templating methods that permit the formation of periodic macroporous solids (pore sizes of a few hundred nm), as well as structures with multiple pore sizes. A range of compositions can be obtained by the following general procedure: In a first step, colloidal crystals are formed by packing uniform spheres (e.g., monodisperse polystyrene or poly(methyl methacrylate) spheres) into three-dimensional (3D) arrays. The 3D colloidal crystals resemble naturally occurring opals. In a second step, the interstitial space is filled by a fluid that is subsequently converted into a solid skeleton. In a final step, the spheres are removed by calcination or extraction, typically creating interconnected voids where the spheres were originally located and a solid skeleton in the location of the former interstitial spaces. The resulting structures can therefore be regarded as inverse replicas of the template array, or inverse opals. Surface modification is possible by methods similar to those outlined for mesoporous sieves, above. Using metal alkoxide or metal salt precursors we have prepared a wide range of periodic macroporous oxides, phosphates, carbonates, metals, alloys and hybrid organosilicates. Silicates with dual pore structures (microporous or mesoporous walls surrounding larger macropores) could be prepared by using multiple templates. By controlling synthesis conditions, it is possible to tailor pore sizes, wall thicknesses, and sometimes the phase of the wall (amorphous or various crystalline phases) according to specific requirements. Often achievement of a pure phase involves balance between temperature, grain growth, and maintenance of an ordered porous structure. On several occasions, purer phases were achieved at higher temperatures, which also led to sintering or particle agglomeration, and therefore loss of ordered macroporosity. We have addressed this issue through the use of chemical additives, modification of template surfaces and template sizes, and through the choice of precursors in titania, zirconia and alumina systems.
We have investigated several potential applications that may benefit from the structure, periodicity, low density, highly accessible surface, and compositional variety of these macroporous solids, including their use as photonic crystals or optical sensors, catalyst supports, sorbents for heavy metals, host materials for drug molecules, bioactive glasses, porous electrodes or electrolytes, and magnetic materials.